Breaking News from 18th International Malignant Lymphoma Meeting, Lugano June 2025
POLARGO Trial Shows Overall Survival Benefit with Pola-R-GemOx in Transplant-Ineligible R/R DLBCL
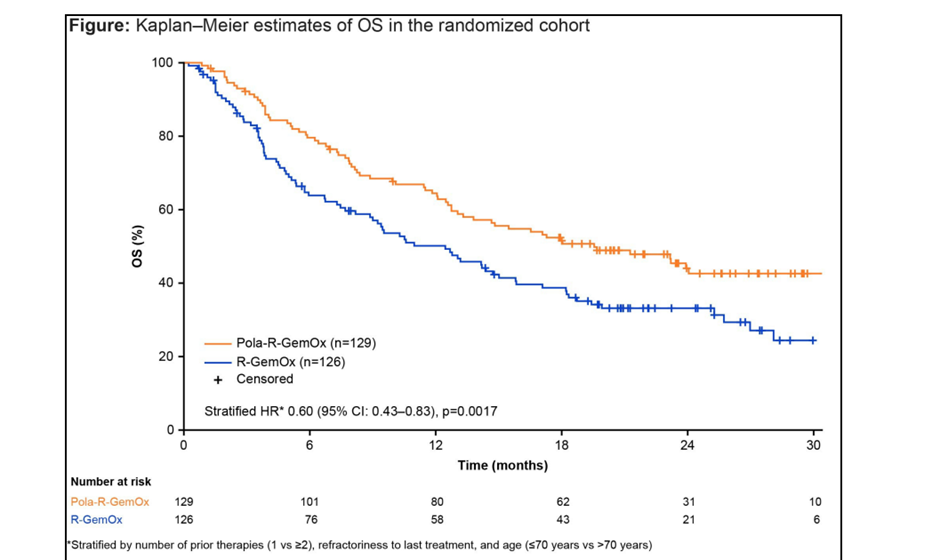
The POLARGO trial (NCT04182204) is a global, randomized, Phase III study designed to evaluate the efficacy and safety of polatuzumab vedotin in combination with rituximab, gemcitabine, and oxaliplatin (Pola-R-GemOx) versus rituximab, gemcitabine, and oxaliplatin (R-GemOx) in patients with R/R DLBCL after at least one prior line of therapy who are ineligible for autologous stem cell transplant.
After a safety run-in phase (n=15), 255 patients were randomized 1:1 to:
- Pola-R-GemOx: polatuzumab vedotin (1.8 mg/kg) + rituximab (375 mg/m²), gemcitabine (1000 mg/m²), and oxaliplatin (100 mg/m²)
- R-GemOx alone (same doses)
Pola-R-GemOx demonstrated a significant survival benefit and 40% reduction in the risk of death over R-GemOx in transplant-ineligible patients with R/R DLBCL. Despite increased rates of peripheral neuropathy and infections, the treatment remained tolerable and effective, offering a valuable alternative that avoids T-cell depletion. These findings support the incorporation of polatuzumab vedotin into GemOx regimens for selected patients, expanding options beyond traditional salvage therapies.